To Simplify International Clinical Trials, Schreiner MediPharm Equips Hanger-Label for Infusion Bottles with Booklet
The specialty label combination of Pharma-Tac and Booklet-Label enables efficient and convenient use of infusion bottles in clinical trials.

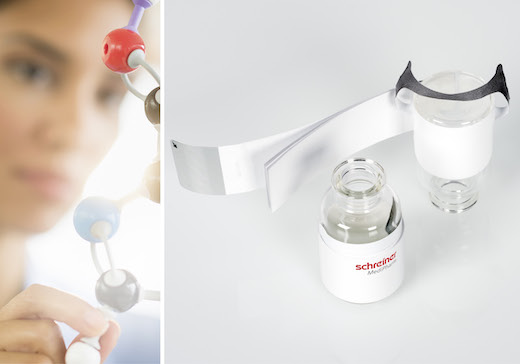
Allowing detailed product descriptions in multiple languages, new Pharma-Tac Plus Label combines infusion bottle hanger with ample marking space.
Schreiner MediPharm, a Germany-based global provider of innovative functional label solutions for the healthcare industry, has developed a new specialty label for infusion bottles to enhance convenience and efficiency in international clinical trials. The new Pharma-Tac Plus Label combines a Booklet-Label with a label-integrated hanger for infusion bottles, creating ample space for comprehensive product descriptions in multiple languages.
The specialty label provides research-based pharmaceutical companies with a marking solution that offers greater flexibility and reliability for clinical trials – especially those conducted in multiple countries or languages.
Schreiner MediPharm’s Clinical Trial Supply Team, which specializes in solutions for clinical research, combined the originial Pharma-Tac label’s strong hanger with a multipage paper booklet. The hanger is an integral component of the label; in a simple, user-friendly fashion, the hanger is separated from the label and folded over to suspend the infusion bottle.
The multipage Booklet-Label is firmly connected to the base label, and can be opened and reclosed via a tab. The customizable solution offers ample space for multilingual product information. The hanger label and booklet combination can be adapted to various vial and bottle sizes, and the number of booklet pages can be tailored to suit specific requirements.
The Pharma-Tac Plus Label with integrated booklet provides pharmaceutical companies and contract research organizations (CROs) with a convenient, flexible labeling solution that efficiently supports international clinical trial efforts. Healthcare professionals benefit from the new label solution as well, since it enables fast and safe administration of infusions, and because including relevant product information in various languages facilitates clinical studies on an international scale.
